This project aims at developping a portable medical device, in order to measure the concentration of the Cystatine marker within blood and saliva, in a less invasive manner than a blood test. This marker helps assess the state of the kidneys in patients suffering from chronical renal disease. The device is destined to be used as a Point-of-care, be it in the doctor’s office, the chemist shop, or at home, so as to provide better and more frequent preventive monitoring of potential complications.
The device exploits the technology developed by the local start-up MagIA-Diagnostics, which specialises in fast and reliable infectious disease screening.
Thanks to the financial subsidies from Région Auvergne-Rhône-Alpes, the CYSTATINE partnership succesfully pushed two major taskforces :
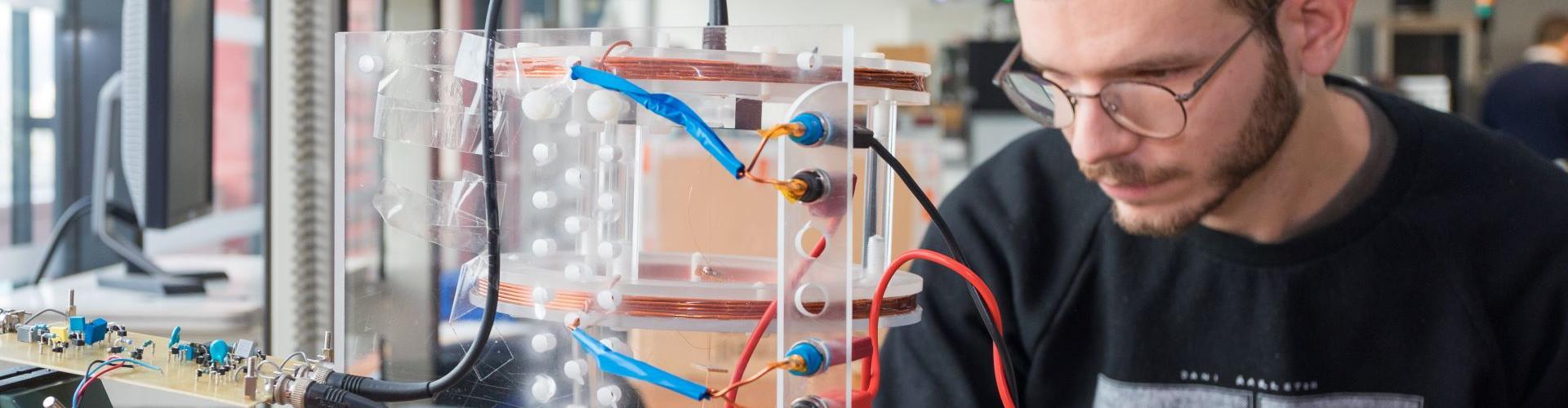
- G2Elab built a first functionnal demonstrator of a simple, portable reader, allowing rapid analysis of samples from a single fingertip blood droplet, using the Magia-Diagnostics technology;
- IAB developped the protocol for effective Cystatine level reading within saliva samples, along with comparative benchmarking against both the standard ELISA method and the reliable lab reader developped by Magia-Diagnostics.
The COVID crisis demonstrated the crucial need for widespread availability of this type of simple, rapid, portable point-of-care device.
Photos :

Functionnal portable reader developped within CYSTATINE.

Optics module for differential epifluorescence image analysis.
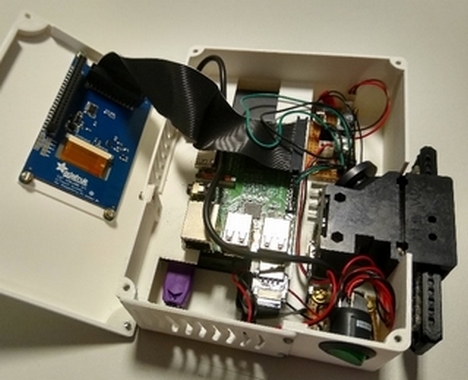
On-board electronics and optics within the portable reader.
Partners :
- G2Elab : Orphée Cugat, Samuel Fumat, Pierre Tacyniak, Celia Mansilla
- IAB : Patrice Marche, Christine Charrat
- Magia-Diagnostics : Sarah Delshadi
Institutions :
L’Institute for Advanced Biosciences IAB, G2Elab, start-up Magia-Diagnostics. The G2Elab team is hosted within CIME-Nanotec and is an active member of FMNT. Academic finance project management Grenoble INP - UGA